
Beckmann Rearrangement
Description: The Beckmann rearrangement is a reaction of oximes that can result in either amides or nitriles, depending on the starting material. (Oximes derived from ketones give amides; oximes derived from aldehydes provide nitriles)
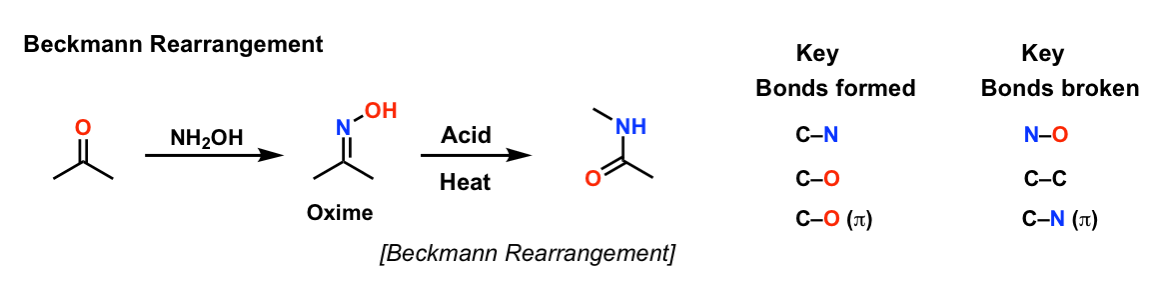
Notes: Note that the first step (formation of the oxime) is technically not part of of the Beckmann rearrangement. The Beckmann is the rearrangement of the oxime to the amide. This is generally achieved through conversion of the oxime oxygen to a good leaving group, followed by heat, which results in an alkyl (or hydride) shift, breaking the weak N-O bond. The second step involves trapping of the carbon with water (forming an amide) or, if a hydride shift occurred, deprotonation of nitrogen to give a nitrile.
Examples:
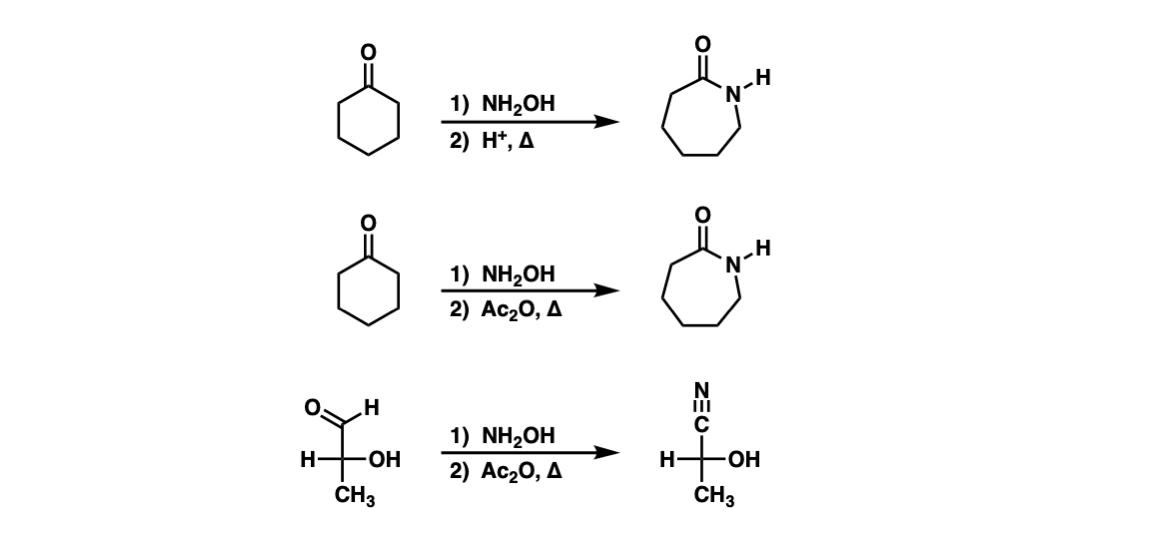
Notes: The Beckmann rearrangement is achieved by converting the oxygen of the oxime to a good leaving group, and then heating. In the first example, treatment of the ketone with NH2OH forms the oxime, and then in step 2, heating with acid leads to the Beckmann rearrangement. In the second example, after formation of the oxime, it is treated with Ac2O (converting the oxime oxygen to an acetate, a better leaving group than OH) and then heating. The third example is a Beckmann rearrangement on an oxime derived from an aldehyde, which results in a nitrile.
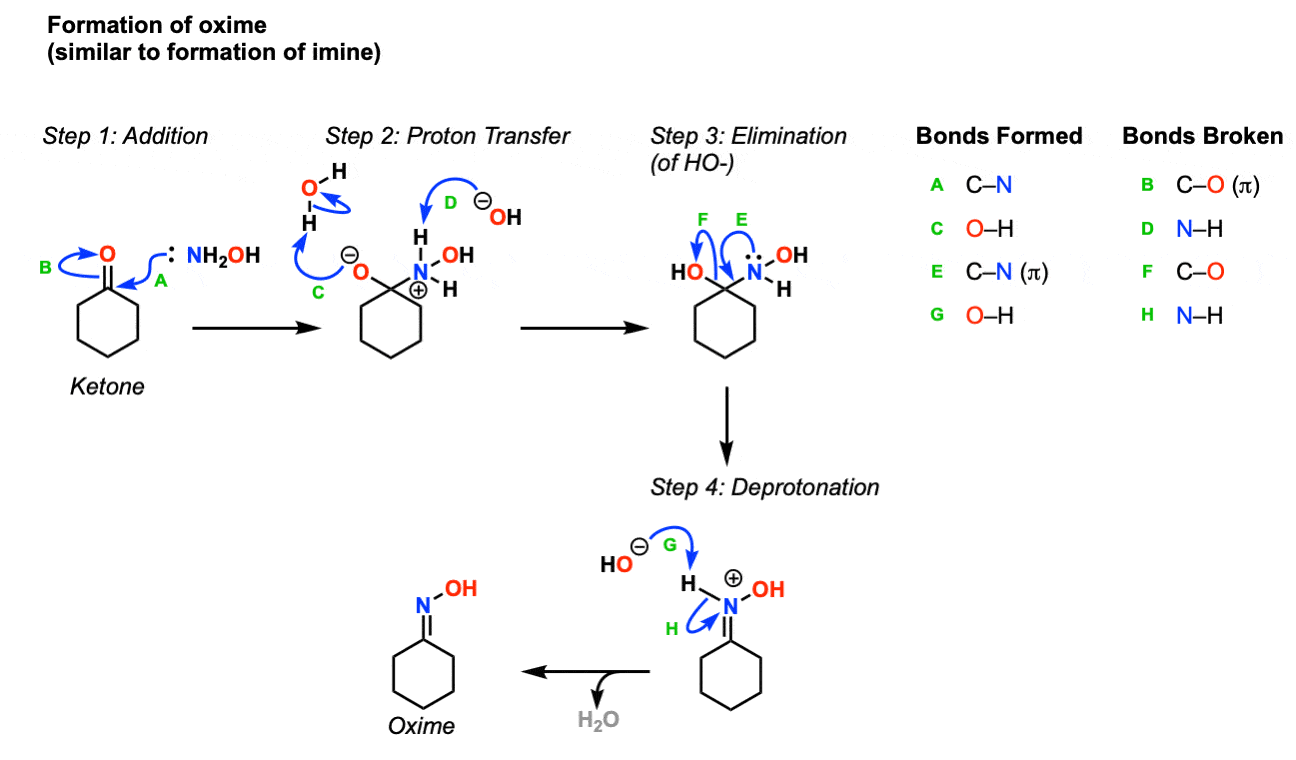
Mechanism: The first step in the process is formation of an oxime from the aldehyde or ketone, which occurs in a sequence similar to formation of imines and hydra zones. In the first step, the nitrogen adds to the carbonyl carbon (Step 1, arrows A and B) followed by proton transfer (Step 2, arrows C and D). Then, the lone pair of nitrogen displaces the hydroxide in an elimination reaction (Step 3, arrows E and F) and finally the nitrogen is deprotonated by base (Step 4, arrows G and H) to give the oxime.
In the Beckmann rearrangement step, the oxime oxygen is protonated by acid (Step 1, arrows A and B). This leads to formation of OH2+, which is a much better leaving group than OH . Now comes the key step, rearrangement. The C-C bond breaks, migrating to the nitrogen, forming a new C-N bond and displacing the water as a leaving group (Step 2, arrows C and D). This forms a free carbocation, which is then attacked by water (Step 3, arrow E). The positively charged oxygen is deprotonated by base (Step 4, arrows F and G) to give the tautomer of the amide. Steps 5 and 6 represent “tautomerization”. First, protonation occurs at nitrogen (Step 5, arrows H, I and J), followed by deprotonation of oxygen (Step 6, arrows K and L). This gives the amide.
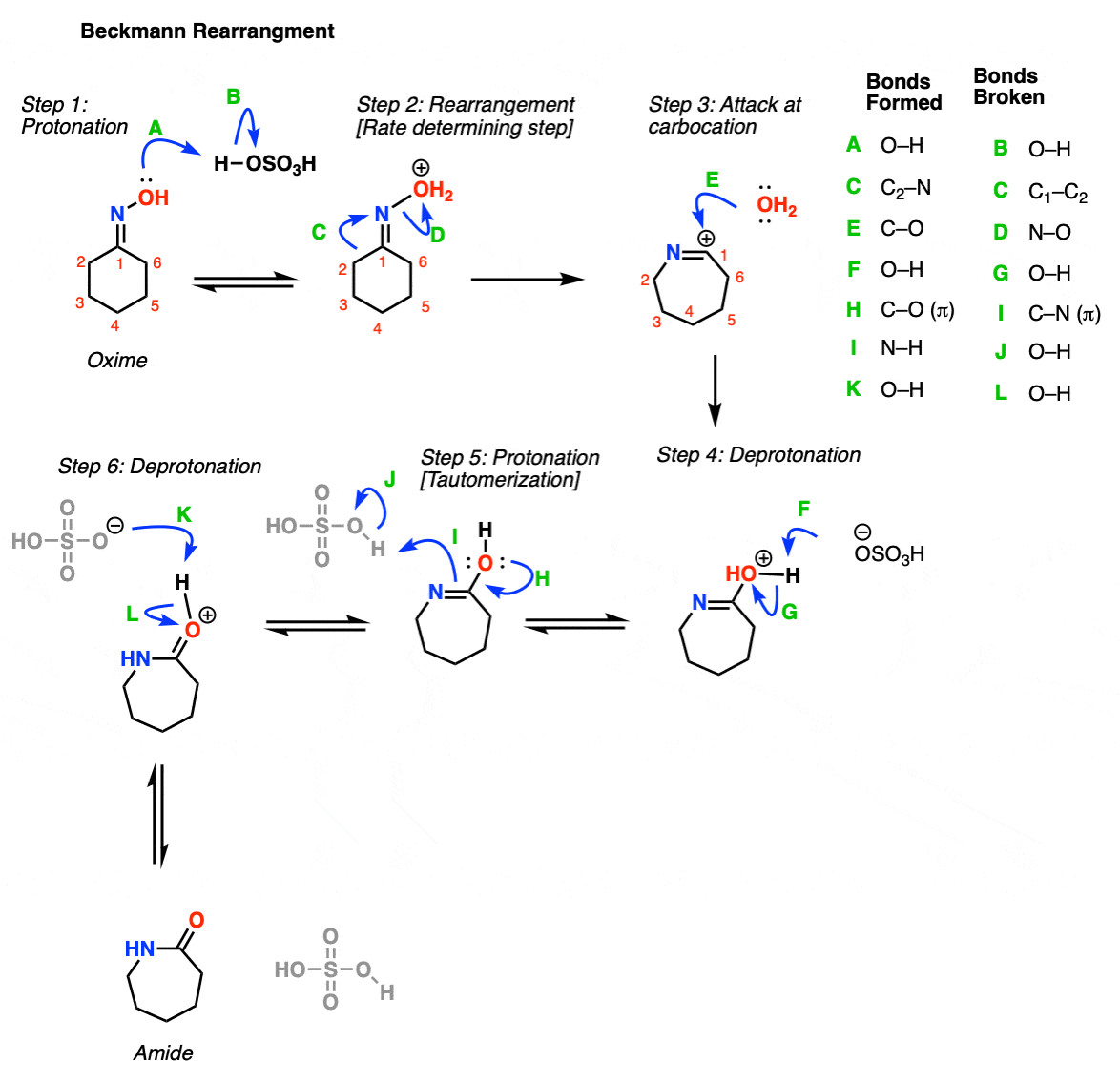
Notes: The key rearrangement step (Step 2) is similar to the rearrangement step in other rearrangement reactions (such as hydride and alkyl shifts, as well as the Curtius, Schmidt, and Wolff rearrangements). This is an alkyl shift, accompanied by loss of the leaving group.
The purpose of the acid is to make the oxygen a better leaving group; the O-N bond is weak.
Note that although HOSO3- is shown as the base here, it’s very possible that other species can act as the base. Also, the ‘proton transfer’ step shown in oxime formation can be shown as two different steps, instead of the one.
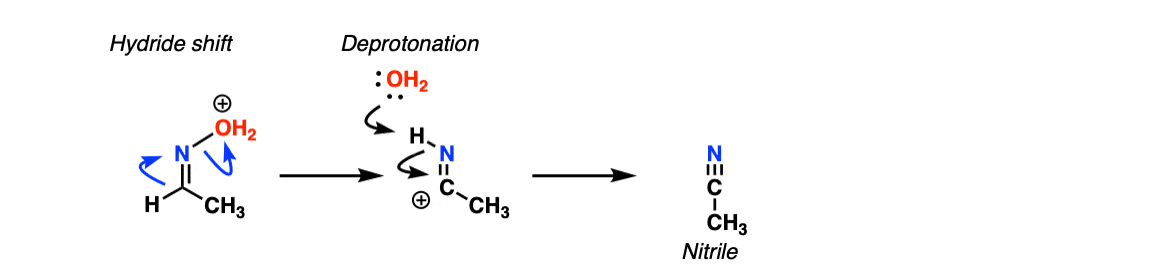
For nitrile formation, the key step is a hydride shift, followed by deprotonation of the nitrogen. This gives the nitrile.
(Advanced) References And Further Reading:
- Zur Kenntniss der Isonitrosoverbindungen Ernst Beckmann Ber. 1886, 19 (1), 988-993 DOI: 10.1002/cber.188601901222 The original paper by Ernst Beckmann on the rearrangement of oximes to amides.
- ε-BENZOYLAMINOCAPROIC ACID C. Eck and C. S. Marvel Org. Synth. 1939, 19, 20 DOI: 10.15227/orgsyn.019.0020 Part B in this reference is a classic and standard Beckmann rearrangement of cyclohexanone oxime to e-caprolactam in Organic Syntheses, a source of reproducible and independently tested organic chemistry experimental laboratory procedures.
- Phosphorus pentoxide-methanesulfonic acid. Convenient alternative to polyphosphoric acid Philip E. Eaton, Glenn R. Carlson, and James T. LeeThe Journal of Organic Chemistry 1973 38 (23), 4071-4073 DOI: 1021/jo00987a028
This paper by Prof. P. Eaton (who is famous for first synthesizing cubane) describes the use of P2O5-MSA as a substitute for concentrated sulfuric acid or PPA (polyphosphoric acid), which are commonly used as media for the Beckmann rearrangement.
- Kinetics and Mechanism of the Beckmann Rearrangement. B. Jones. Chemical Reviews 1944 35 (3), 335-350 DOI: 10.1021/cr60112a001 An early review which describes work done towards understanding the mechanism of the Beckmann rearrangement.
- Establishing a Molecular Mechanism for the Beckmann Rearrangement of Oximes over Microporous Molecular Sieves Ana B. Fernández, Mercedes Boronat, Teresa Blasco, Avelino Corma Angew. Chem. Int. Ed. 2005, 44 (16), 2370-2373 DOI: 10.1002/anie.200462737 More recent work on the mechanism of the Beckmann rearrangement, using computational methods and solid-state NMR spectroscopy.
Real-Life Example:
Org. Synth. 1939, 19, 20
DOI Link: 10.15227/orgsyn.019.0020
Link nội dung: https://sgk.edu.vn/o2-ra-p2o5-a72658.html