
Experiment on the reaction of Sodium sulphate with barium chloride in the form of their solutions in water and classify them as physical or chemical changes
A physical change occurs when there is no change in the composition of a substance and no change in the chemical nature of the substance.
The interconversion of state occurs during physical change.
SOLID ⇄ LIQUID ⇄ GAS
A chemical change is a change that causes a change in the chemical properties of matter, resulting in the formation of a new substance. As an example, consider the burning of oil or fuel.
Heat is evolved or taken in, the formation of bubbles, gas, and fumes, as well as a change in the colour of the reactants, can take place when they form a product.
Reactants → Products
A + B → C (Chemical reaction)
Table of Contents
- Aim
- Materials Required
- Theory
- Procedure
- Observation
- Result
- Precautions
- Frequently Asked Questions - FAQs
Aim
To carry out the reaction of Sodium sulphate with barium chloride in the form of their solutions in water and classify them as physical or chemical changes.
Materials Required
Test tube, Test Tube Holders, An Aqueous solution of Sodium Sulphate and Barium
Chloride
Also Read: Sodium sulphate with barium chloride in the form of their solutions in water Viva QuestionsTheory
On mixing the solutions of sodium sulphate and barium chloride, a white
precipitate of barium sulphate is formed, which in insoluble in water.
In this reaction, the white precipitate of BaSO4 is formed by the reaction of SO42- and
Ba2+. The other product formed is sodium chloride which remains in the
solution. Such reactions in which there is an exchange of ions between the
reactants are called double displacement reactions.
Procedure
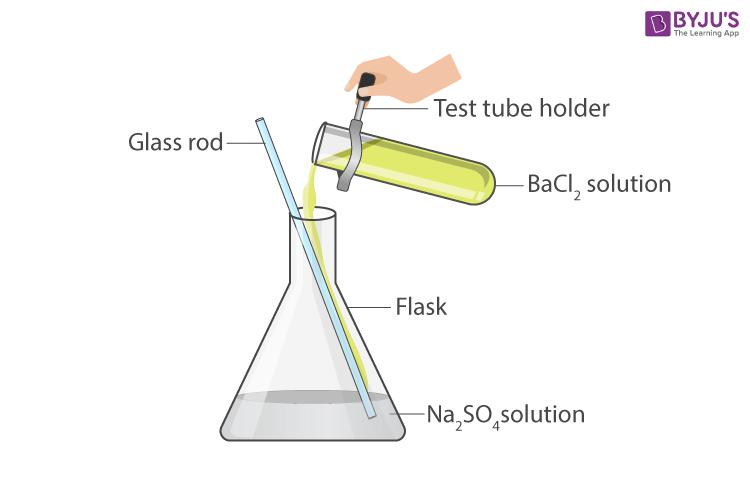
- Take a small amount of sodium sulphate solution in a test tube.
- Add a small amount of barium chloride solution to it.
- Stir the solution in the flask with the help of a glass rod and leave it undisturbed for some time.
- Add a small amount of dilute hydrochloric acid to the flask.
Observation
- Both Barium chloride and Sodium sulphate are colourless.
- A white precipitate of barium sulphate can be observed. It is insoluble in dilute hydrochloric acid.
Result
- The reaction of Na2SO4 (aq) and BaCl2 (aq) produces an insoluble white precipitate of BaSO4.
- This indicates that it is a double displacement reaction.
- Since the products formed are different from the reactants in chemical properties and composition. Thus, it is a chemical change.
Precautions
- Use only a small amount of the chemicals.
- After you’ve finished the experiment, wash your hands with soap.
- Do not try to taste or touch the chemicals.
- While combining the solutions in the mixture pour sodium sulphate first and then slowly add barium chloride to it.
Link nội dung: https://sgk.edu.vn/bacl2-na2s-a71981.html