What is PBr3?
Phosphorus tribromide, also known as PBr3, is a colourless liquid with the formula PBr3. Due to hydrolysis, the liquid emits a pungent odour in moist air.
Red phosphorus is treated with bromine to produce PBr3.
2P + 3Br2 → 2PBr3
To prevent the formation of PBr5, an excess of phosphorus is used.
The structure of Phosphorus Tribromide is a pyramidal structure with sp3 hybridization.
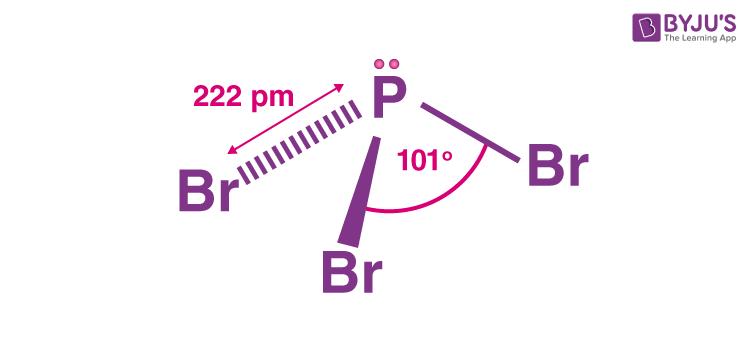
Table of Contents
- Reactions of PBr3
- PBr3 Mechanism
- Applications of PBr3
- Frequently Asked Questions - FAQs
Reactions of PBr3
1. Phosphorus tribromide (PBr3) can be used to convert primary and secondary alcohols to alkyl bromides.

2. Phosphorus Tribromide PBr3 converts carboxylic acid to acid bromides.

3. Phosphorus Tribromide PBr3 reacts with water to form phosphorous acid (H3PO3) and HBr.
PBr3 + H2O → H3PO3 + HBr
Read more: Phosphorus tribromide (PBr3)
PBr3 Mechanism
The mechanism involves the electrophilic phosphorus first activating the alcohol oxygen (to form a good leaving group), followed by an SN2 substitution at the alcohol carbon.
The SN2 substitution step ensures that the reaction works well for primary and secondary alcohols but fails for tertiary alcohols. If the reacting carbon centre is chiral, the reaction usually takes place with configuration inversion at the alcohol carbon, as is typical of an SN2 reaction.

This reaction can also be carried out in pyridine, a weak base. Although the first step in the above mechanism is reversible, subsequent deprotonation by pyridine prevents the reverse reaction from occurring, thereby assisting in product formation. The mechanism for pyridine is also depicted below.

Read more: Lewis structure pf PBr3
Applications of PBr3
- Phosphorus tribromide is primarily used in the conversion of primary or secondary alcohols to alkyl bromides.
- PBr3 typically yields higher than hydrobromic acid and avoids problems with carbocation rearrangement—for example, neopentyl bromide can be made from the alcohol in 60% yield.
- PBr3 acts as a catalyst for carboxylic acid-bromination.
- They serve as intermediates in the Hell-Volhard-Zelinsky halogenation process. PBr3 first reacts with the carboxylic acid to form acyl bromide, which is more reactive to bromination.
- Phosphorus tribromide is commercially used in the production of pharmaceuticals such as alprazolam, methohexital, and fenoprofen.
- It is also a powerful fire suppression agent sold under the brand name PhostrEx.


