What is Acetylene?
C2H2 is the simplest alkyne chemical compound with the chemical name Acetylene.
Acetylene is also called Ethyne or Narcylen or Vinylene. It is widely used as a chemical building block and as a fuel. In its pure form, it is unstable and is handled as a solution. It is an unsaturated compound the two carbon atoms in it are linked together with a double bond.
Table of Contents
- Acetylene Structure
- Recommended Videos
- Properties of Acetylene
- Chemical Properties of Acetylene
- Acetylene Uses
- Production of Acetylene
- Health Hazards
- Frequently Asked Questions - FAQs
Vinylene is a colourless gas which has a mild ether-like odour. It is easily soluble in water, chloroform, acetone, and benzene. It is slightly soluble in carbon disulfide and ethanol. It is lighter when compared to air and easily ignites. Prolonged exposure to heat or fire can rupture containers violently.
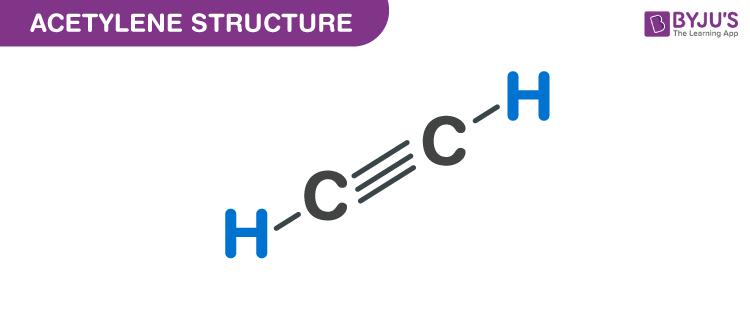
Acetylene Structure - C2H2
Recommended Videos
Properties of Acetylene - C2H2
C2H2 Acetylene Molecular weight of C2H2 26.038 g/mol Density of Acetylene 1.097 kg/m3 Boiling point of Acetylene -84.7°C Melting Point of Acetylene −80.8 °CChemical Properties of Acetylene (C2H2)
1. Reaction with HBr
When acetylene is treated with HBr, the product is Ethylidene bromide.
2. Reaction with sodium metal
Sodium reacts with acetylene to produce sodium hydro acetylide and hydrogen.
Na + C2H2 → NaHC2 + H2
3. Reaction with HCl
When acetylene reacts with HCl, CH3CHCl2 is formed.
Acetylene Uses
- Acetylene is used in brazing.
- Used in the glass industry.
- Used in the manufacturing of synthetic rubber.
- Used in soldering metals.
- Used as an additive to preserve food.
- Used to precipitate metals.
- Used to manufacture acetic acid.
- Used as a feedstock in the manufacturing of acrylonitrile.
- Used in carburization of steel.
- Used as a fuel additive.
Production of Acetylene
Since the year 1950, this compound has been synthesized by the partial combustion of CH4 (methane). Around 4,00,000 tonnes were produced until 1983.
In the year 1862, it was prepared by a reaction which was discovered by Friedrich Wohlerthe. Hydrolysis of calcium carbide reaction is as follows:
CaC2 + 2H2O → Ca(OH)2 + C2H2
The above reaction takes place at an extremely high temperature approximately 2000 °C, with the use of an electric arc furnace.
Health Hazards
People who come in contact with this compound may suffer from headaches, loss of consciousness, and dizziness. Death due to choking can occur if a higher percentage of Ethyne is present in the air.
Learn more about the Structure, physical and chemical properties of C2H2 from the experts at BYJU’S.


